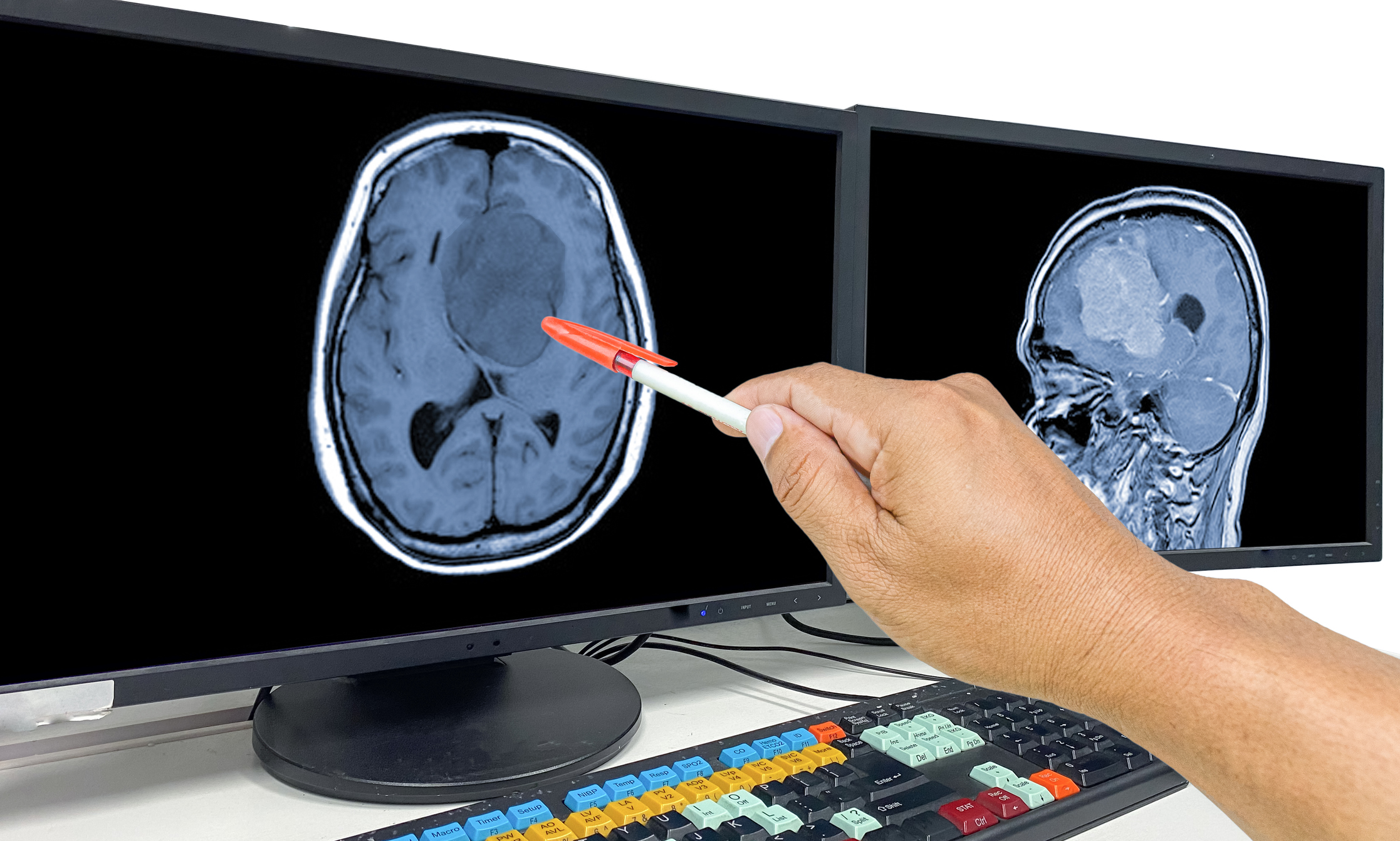
When selecting birth control, understanding potential risks is crucial. While Depo-Provera has been a popular contraceptive for its ease of use, emerging studies indicate a disturbing connection: prolonged use of Depo-Provera may increase the risk of developing meningiomas, a type of brain tumor.
This discovery has raised significant concerns about the drug's safety and the importance of informed consent for patients.
What Is a Meningioma?
A meningioma is a tumor that develops on the meninges, the protective membranes surrounding the brain and spinal cord. While these tumors are often benign, their location can cause severe symptoms, including:
Chronic headaches
Blurred or double vision
Seizures
Cognitive impairment
Meningiomas can grow large enough to require invasive treatments, including surgery or radiation therapy, significantly impacting the quality of life. Learn more about meningiomas from the American Brain Tumor Association.
How Depo-Provera May Increase Meningioma Risk
A growing body of research has highlighted a link between prolonged Depo-Provera use and the development of meningiomas. This risk appears to be associated with the drug’s hormonal ingredient, medroxyprogesterone acetate (a synthetic form of progestin).
Key Findings from Recent Studies:
Long-term use is a major factor: Patients who received Depo-Provera injections for extended periods had a higher likelihood of developing meningiomas.
Higher dosages amplify the risk: Those exposed to higher cumulative doses were at greater risk, underscoring concerns about the drug’s long-term safety.
The connection between synthetic hormones like those in Depo-Provera and tumor growth has been explored in medical journals. For additional context, visit the National Center for Biotechnology Information.
Did the Manufacturer Fail to Warn Patients?
Pharmaceutical companies have a responsibility to disclose known risks associated with their drugs. Lawsuits allege that Depo-Provera’s manufacturer failed to:
Provide adequate warnings about the potential for meningioma development.
Conduct sufficient studies to understand the risks of long-term use fully.
If you’ve developed a meningioma after using Depo-Provera, you may be eligible to pursue compensation for medical bills, lost wages, and emotional distress through a mass tort lawsuit.
What to Do if You’re Affected
If you or a loved one have been diagnosed with a meningioma and have a history of Depo-Provera use, take the following steps:
Document your medical history: Include records of Depo-Provera use and any symptoms or diagnoses related to brain health.
Seek legal advice: An experienced mass tort attorney can evaluate your case and explain your legal options.
Act promptly: Time limits, known as statutes of limitations, may apply to your case.
Learn how to report adverse medication reactions by visiting the FDA MedWatch Program.
Why Choose Kershaw Talley Barlow?
At Kershaw Talley Barlow, we are dedicated to holding pharmaceutical companies accountable when they fail to protect patients. With extensive experience in mass tort litigation, we provide compassionate representation for victims harmed by unsafe drugs, including those affected by Depo-Provera.
Our team is actively investigating meningioma cases and offers personalized legal support to help you navigate your claim.
Take the First Step Toward Justice
If you’ve experienced serious health complications linked to Depo-Provera, you deserve answers—and justice. Contact Kershaw Talley Barlow today at (916) 520-6639 for a free consultation. Our experienced attorneys are here to help you pursue compensation and hold the responsible parties accountable.
For additional information about drug safety and patient advocacy, visit the National Women’s Health Network.